The power of hydrogen, or pH (also called the potential of hydrogen), measures the concentration of hydrogen ions in a solution. The formula for calculating the pH of a liquid is:
pH = -log [H⁺]
A higher pH indicates lower acidity and higher alkalinity in the solution. Conversely, a lower pH means higher acidity and lower alkalinity.
How does power of hydrogen (pH) affect humans?
The concentration of hydrogen ions in a solution greatly affects humans. This concentration determines whether a substance is acidic or basic. But why should we care about the acidity of things? Highly acidic substances can harm our skin and internal organs if we come into contact with them. For example, battery acid is highly acidic and can cause severe damage upon contact.
Now, think about it.
Why does acidic stuff burn your skin?
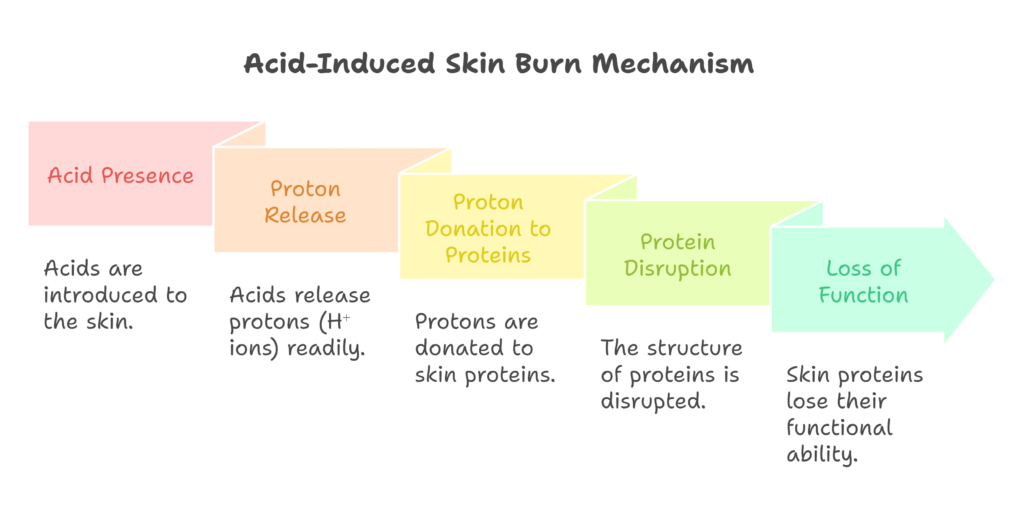
Acids cause burns primarily due to their protons, or free H⁺ ions, which are loosely bound to an electronegative atom within the acid. Most acids consist of an electronegative atom bonded to a hydrogen ion or proton. These electronegative atoms tend to release the proton readily if it finds a lone electron pair to stabilize its positive charge.
In this process, the electronegative atom bonds with a more positively charged entity that stabilizes it more effectively. Consequently, the acid donates the lone hydrogen ion to the nearest lone electron pair. This aligns with the Lowry-Bronsted definition of acids, which states that acids donate protons.
For this reaction to occur, a lone electron pair must be available. Acids donate H⁺ ions to obtain electrons, but how does this relate to the sensation of burning? Specifically, why do strong acids cause more severe burns?
Strong acids release their protons more easily, which increases their capacity to cause burns. Our bodies are made up of proteins, which fold into precise structures to function correctly. When these structures are disrupted, proteins lose their ability to function. When a strong acid comes in contact with skin, it donates protons to the proteins in the skin.
Protein unfolding
Proteins accept these protons due to the presence of carboxylate groups, which carry a negative charge. This negative charge participates in hydrogen bonds and salt bridges, which contribute to the stability of the protein’s structure. When the carboxylate group accepts a proton from the acid, it loses its negative charge, which disrupts the salt bridges and hydrogen bonds. As a result, the protein loses its secondary and tertiary structures and collapses, becoming unfolded.
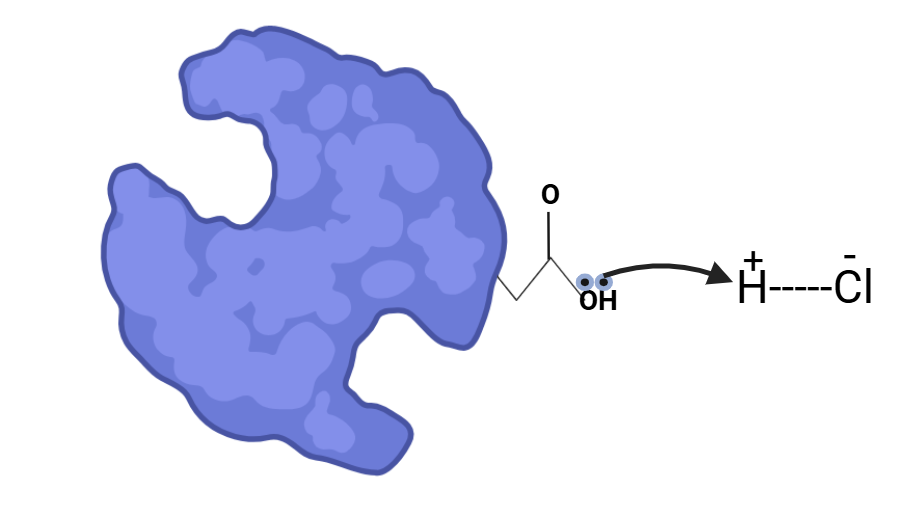
The lone pair of electrons from the carboxylate group of the protein scavenges protons from the acid, causing the collapse of the protein’s secondary structure. This collapse disrupts the protein’s function, as the secondary structure is essential for its proper activity.
When this structural collapse occurs, the cells fill with nonfunctional proteins. We experience this process as a burn—acid protons alter enough proteins in the cells, causing them to clump into nonfunctional masses. This is the damage we see when tissue is burned by a strong acid.
How to measure acidity and basicity using power of hydrogen?
The mathematical value of power of hydrogen (pH) is determined by the formula:
pH = -log [H⁺]
But how do we measure hydrogen ion concentration in a solution? One way to do this is by using a pH meter, also known as a potentiometric pH meter. We will explore why it has this name shortly.
To measure pH, immerse the pH meter into the solution. The meter measures the pH directly and displays the result on a digital screen, showing the solution’s pH value.
A higher pH or power of hydrogen indicates lower acidity and higher alkalinity in the solution.
Conversely, a lower pH means higher acidity and lower alkalinity.
What is a pH meter?
A pH meter consists of two electrodes: a glass electrode and a reference electrode. Both electrodes have a silver wire coated with silver chloride. The silver metal in the electrode can lose electrons and become a positively charged silver ion (Ag⁺). This reaction is reversible, meaning the silver ion can also gain electrons and form metallic silver.
Another important reaction is the interaction between silver ions and chloride ions to form silver chloride (AgCl). The reference electrode is filled with saturated KCl, while the glass electrode contains 0.1M HCl.
The glass electrode has a thin glass bulb made of silica (SiO₂). The oxygen atoms bound to the silicon atoms on both the inner and outer surfaces of the glass are negatively charged. These negatively charged oxygen atoms attract positively charged hydrogen ions (H⁺). When bound by H⁺ ions, a hydrated gel layer forms on both sides of the glass bulb. The hydrogen ions cannot pass through the glass layer; they can only bind to the surface of the glass bulb.
The hydrated gel layer is about 10 nm thick, while the glass layer itself is approximately 0.1 mm thick. The glass electrode half-cell is connected to the reference electrode. The reference electrode has a porous ceramic plug, which allows the diffusion of ions and helps complete the circuit.
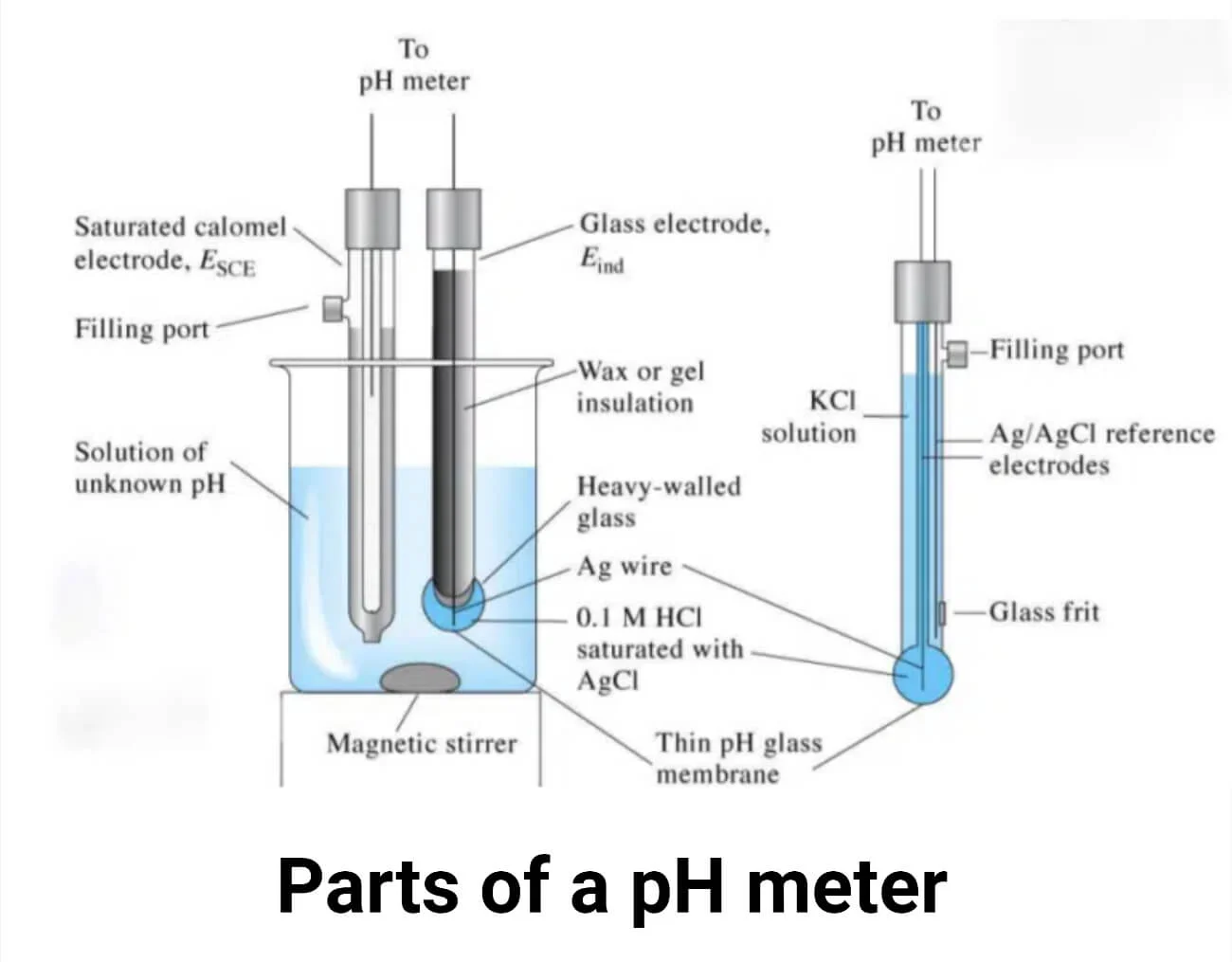
How does a pH meter work?
Power of Hydrogen in acidic solution
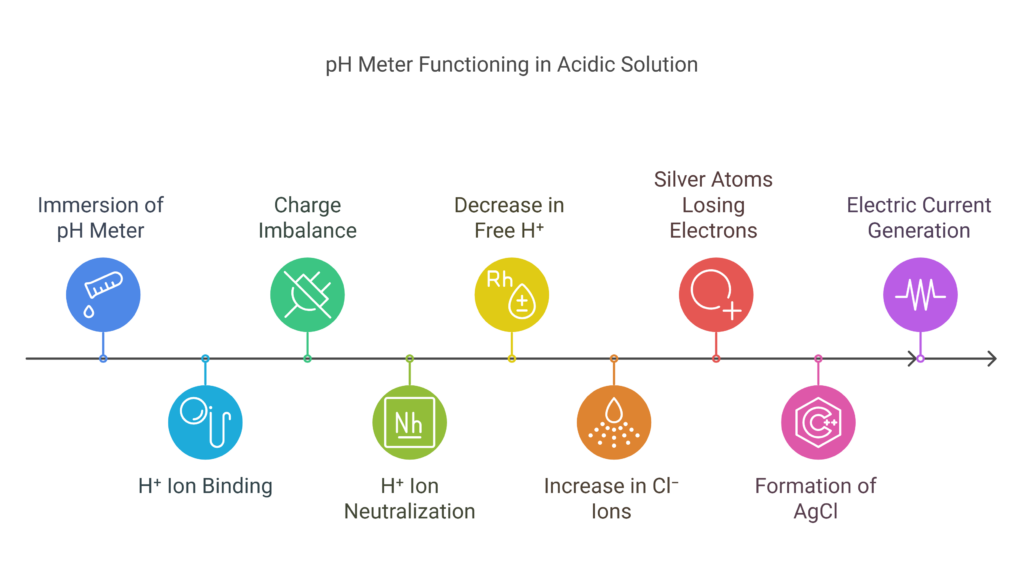
When the solution is acidic and you immerse the pH meter in it, more H⁺ ions bind to the outer layer of the glass bulb. This causes the region outside the glass electrode to become more positively charged, while the inner layer, with fewer H⁺ ions, remains less positively charged.
The inner solution of the glass electrode contains HCl, which releases H⁺ ions. These ions help balance the charge across the glass bulb by neutralizing the charge from the binding of hydrogen ions in the outside solution. This process causes the concentration of H⁺ ions inside the glass electrode to decrease slightly.
However, the total number of H⁺ ions inside the glass electrode remains constant. As the H⁺ concentration is constant, the pH also remains constant. But since the free H⁺ concentration decreases, the concentration of free Cl⁻ ions increases.
At the silver wire, silver atoms lose electrons and bind with chloride ions to form silver chloride (AgCl). The electrons freed from the silver atoms are present in the silver wire of the electrode. These electrons create an electric current, which generates a voltage in the silver wire. The device measures the change in potential of the glass electrode and silver wire against the reference electrode, which serves as a standard for the glass electrode’s potential. Visit this site for a video explanation.
Power of Hydrogen in Alkaline solutions
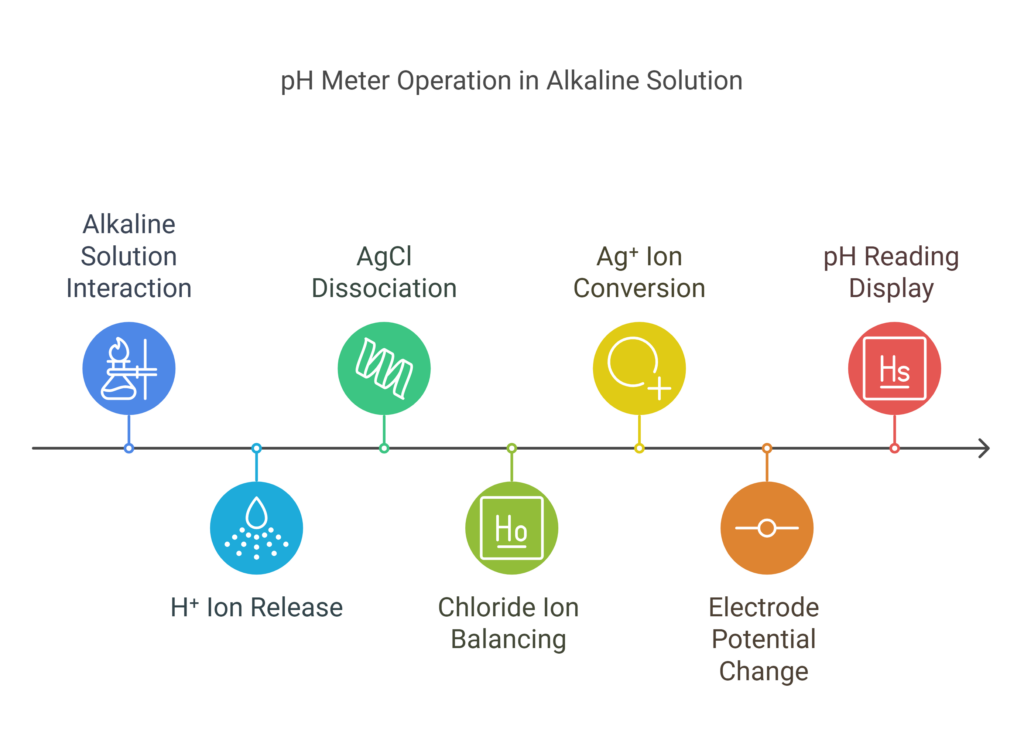
In an alkaline solution, the concentration of H⁺ ions in the outer layer of the glass electrode is lower than the concentration inside the glass electrode. The outer region becomes less positively charged, while the inner layer, with more H⁺ ions, becomes more positively charged. To balance the net charge, H⁺ ions from the inner layer are released into the solution inside the glass electrode. This increases the concentration of free H⁺ ions in the glass electrode. To neutralize this excess of H⁺ ions, AgCl from the silver wire dissociates into silver and chloride ions. The chloride ions balance the extra hydrogen ions in the solution.
Now, excess Ag⁺ ions are present in the solution. These ions accept electrons from the electrode and gain electrons to become metallic silver, which then deposits onto the electrode. Since the glass electrode now gives electrons, it becomes positively charged, and the potential changes. This change in potential is measured in reference to the reference electrode.
In both acidic and alkaline solutions, the potential difference is a measure of the power of hydrogen or pH value. The device translates this value into a pH reading, which appears on the digital screen. The pH meter provides an accurate reading when properly calibrated. Visit this site for a video explanation.
What is the pH of Water?
Water has a pH of roughly around 7. Pure water has a pH of 7, and, generally, rainfall is somewhat on the acidic side (a bit less than 6).
Precautions
- To ensure accurate readings, make sure that the probe of the pH meter does not touch any surfaces.
- Clean it with distilled water before and after using it in a new solution.
Where to buy a pH meter?
We at Labkafe provide the best quality lab equipment, including pH meters calibrated for daily laboratory use.
Contact the experts at Labkafe today for pH meters and other lab equipment






Leave a Reply