Working in a chemistry laboratory with lots of acids and bases? Great stuff, but how do you know what you made is acidic or alkaline? You can hardly tell from the looks, and performing individual tests every time is insane. This is where an universal indicator comes in handy, which can detect how acidic or basic a solution is very reliably.
To explain what an universal indicator is, we have to talk about pH values a bit. The reason for that is that an indicator changes color depending upon the pH value of a watery solution . Since this pH value is what exactly determines how an indicator will behave, let’s learn about this first, shall we?
What is pH and How it Works
Simply put, the pH value of a watery solution is a number indicating the amount of hydrogen ions present in that solution. Now, we can’t exactly tell how many hydrogen ions are present in the solution, can we? So, instead, what we measure is the power of hydrogen (or the potential of hydrogen) in the solution in question.
This can be done with a proper laboratory setup, testing the solution’s electrical charge at the positive pole in an electrolytic cell. But that kind of stuff is not only costly, but it is prohibitively complicated as well. Also you may not always have the setup. This is why pH indicators are used.
Why measure hydrogen ions at all? You see, acids and bases orbit hydrogen in a special way. Acids all release hydrogen ions in a solution; therefore, the more hydrogen ions are present in a solution, the more acidic it would be. On the other hand, bases release other cations like sodium or ammonium in a water solution, suppressing the natural hydrogen ions in water. So, the less hydrogen power in a solution, the more alkaline it is.
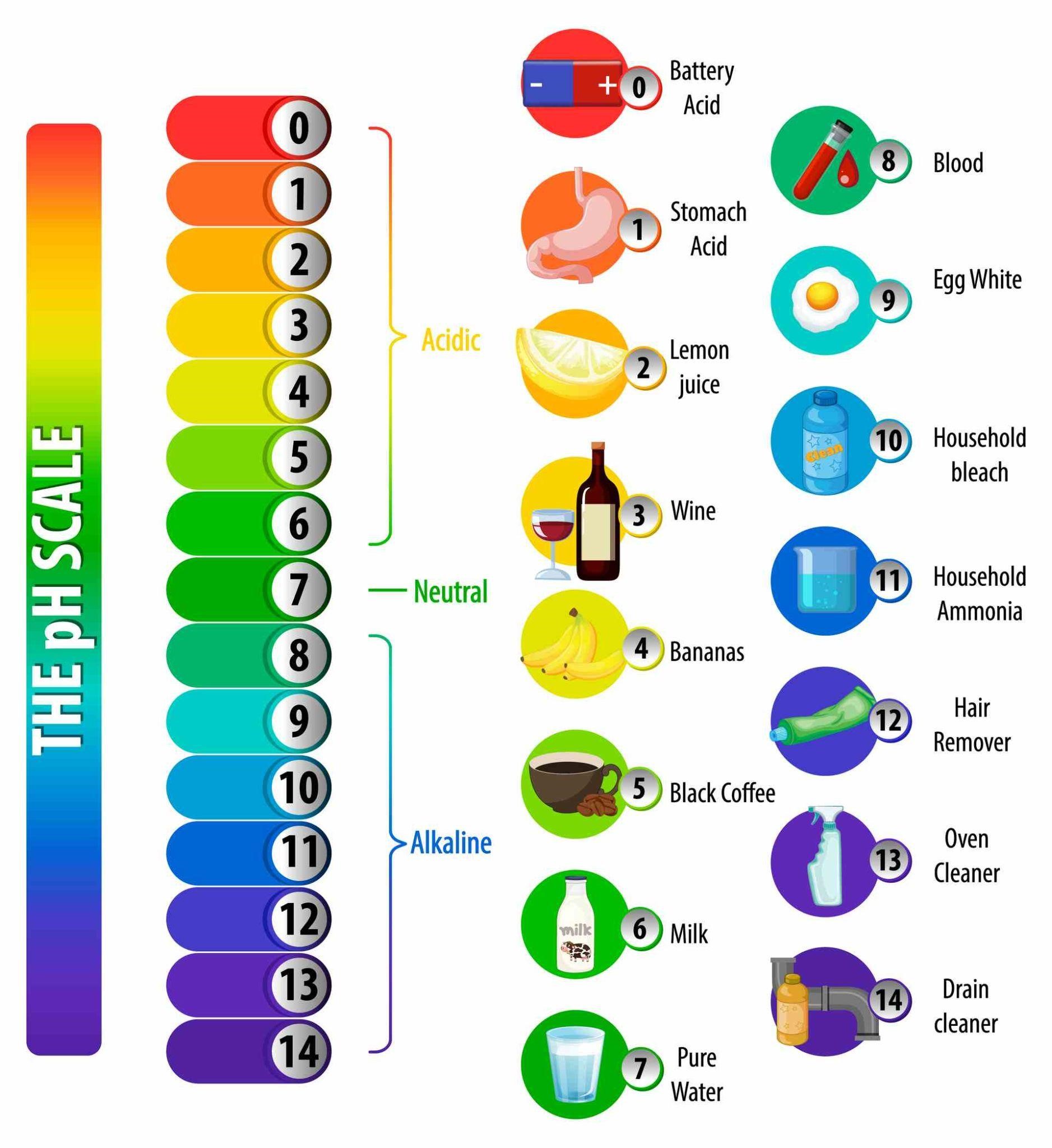
And the pH scale measures exactly that. The pH scale ranges from 0 to 14, each number denoting a specific hydrogen ion potential in a given solution. The pH 7 is hypothesized to be the perfectly medium pH value, meaning that a solution of pH 7 is completely balanced between acidic and basic. Anything below 7 is acidic in increasing strength, and vice-versa for bases.
This is why the best DM water would have a value as close to 7 as possible. Normal drinking water out of RO machines range around pH 7.2 to 7.5. You should note that all of this depends upon the temperature of the solution ‒ with an increase in heat, the pH value of the same solution can drop.
What is an Acid/Base Indicator
An acid-base indicator is a chemical that changes color depending upon the amount of hydrogen ions present in a solution. There are actually many such indicators out there, ranging from phenolphthalein to litmus paper to methyl blue. They act in a given range of pH values. Generally, we use either indicator solutions that we drip in a solution; or pH papers that we drip the solution on.
These chemicals are called halochromic compounds, meaning that they exchange electrons with H+ or H3O+ ions. When those electrons jump around in their orbits, they absorb certain colors of the light spectrum and reflect/let pass the rest, so we see a change of color. Learn why things have colors here .
Either way, when the color changes in a certain direction, based upon the nature of the indicator you will know the acidity or basicity of your solution. At least, you will know whether it is acidic or basic. How much? Well, that’s where we come to the universal indicator.
What is the Universal Indicator
The problem with normal indicators is that they work within a very specific range of pH values only. Using them, you can determine whether your solution is acidic or basic; but not how much. Also, they wouldn’t work on some specific solutions.
Take Methyl Red for example. It turns red under pH 4.4 and yellow above pH 6.2, but anything beyond those values remains more or less the same. If, suppose, you were working with a strong acid and you were expecting to reduce its acidity a little bit, then methyl red won’t be able to help you figure that at all.
That is to say, a litmus paper can tell us that both vinegar and sulphuric acid are acidic in nature, but it can’t tell us which one is the stronger acid. From experience we know that sulphuric acid is way stronger than plain old vinegar (we actually consume vinegar but can’t consume sulphuric acid), but what good is an indicator if it can’t tell us that simple fact?

This is why scientists prepared the Universal Indicator. Patented by Yamada in 1933, this is a mix of various different indicators in different strengths that, working together, gives finely defined colors at different pH values ranging almost the whole scale. Using the universal indicator you can easily determine the change of acidity or basicity in your solution gradually. It is best used in colorless solutions.
Universal Indicator Formula
Most of the universal indicators available in market these days are close variations of Yamada’s original formula, which was mixed thusly:
Water + 1-propanol + phenolphthalein + sodium hydroxide + methyl red + bromothymol blue + sodium bisulphite + thymol blue
These are the components of the universal indicator used mostly everywhere. Note that this mixture is very flammable, so take extra care not to use it near open flames or hot stuff. Also, the liquid is not supposed to be used in boiling or chilled solutions ‒ let it balance off first to room temperature. Adding to the list of precautions while using the universal indicator, it is also harmful to skin and is poisonous, so make sure you never touch it with bare skin, or inhale it or, god forbid, ingest it!
Universal Indicator Colors
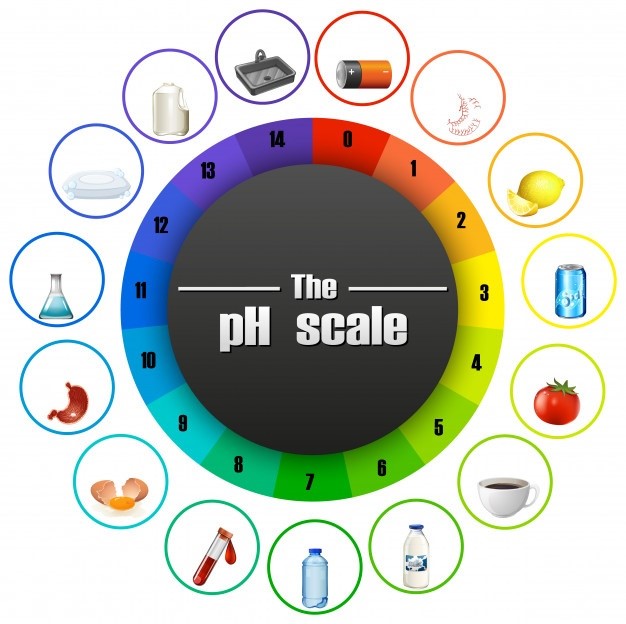
As we have said earlier, the universal indicator is so sensitive to hydrogen atoms in a solution that it changes color rapidly at once at any change in their concentration. In strong acids (pH 0 to 3) it will be red, in weak acids (pH 3 to 6) it will be orange to yellow, in neutral solutions it will be variations of green, in weak base (pH 8 to 11) it will be blue, and in strong bases (pH 11+) it would be indigo to violet to fuchsia.
Note: you can expect the above colors at room temperature (25℃).
The pH Scale Colors with Universal Indicator
The above scale will tell you certainly which color you may expect at which level of pH value. However, don’t expect an absolute certain color; of course things differ depending upon the purity of the solution, the concentration of it, and the nature of the indicator itself ‒ different brands may give slightly different shades. We at Labkafe determined the pH color chart above by using a Nice brand Universal Indicator solution , at room temperature, using medium concentration solutions.
Additionally, we recommend you to see this picture at Wikipedia depicting excellent levels of indication using the universal indicator.
How to Use the Universal Indicator
There are two physical forms in which you can use the universal indicator ‒ paper strips or liquid solution. They have different uses, as follows.
- Paper Form: this is the common pH paper we can find in most shops. Basically a strip of colored paper, it would change color as it comes into contact with an acidic or basic solution. This is useful when the solution itself is of dark color, or if the test matter is quite thick. All you have to do is to lay the paper on the surface of the solution, and only the paper changes color. Additionally, this does not disturb the test subject itself. But don’t be too certain about the color to determine the exact pH value of the solution.
- Solution Form: When you have a colorless solution and are not bothered by disturbing it, then use the universal indicator solution to determine the pH value of the solution as closely as possible. Generally, you can add 3-4 drops of the indicator to 5 ml of the sample solution. This presents a highly accurate result. Of course, this idea goes out the window if the solution already has its own color.
Many industries use the universal solution to determine the quality of their products. Any industry dealing with chemicals falls into this category, mostly chemical products and pharmacy product manufacturers. All chemistry and bio labs will have large stocks of the universal solution in store, and you may find some at hospitals as well.
Additionally, pH value testing is strongly indicative at soil culture, groundwater and other water quality testing, in water pumps and storages, agriculture, and much more. In construction, pH value of the soil is highly important since acidic soil can and will corrode reinforced concrete. Since the acidity of the water determines the life in it, many fisheries and marine biologists regularly use the universal indicator too.










Leave a Reply